Congratulations to
Sandwich molecule

Picture: Peyker / Shutterstock.com

Picture: Peyker / Shutterstock.com
Ernst Otto Fischer was awarded the Nobel Prize in Chemistry in 1973 for a seemingly impossible molecule called ferrocene. Working at what is now the Technical University of Munich (TUM), Fischer discovered its sandwich-like structure with an iron atom as the filling. This breakthrough helped scientists deepen their understanding of how atoms can bond to each other.
Ernst Otto Fischer could himself barely believe the results of his experiments in 1952 – but no other explanation was possible. An iron atom really was layered between two five-membered hydrocarbon rings, like the filling in a sandwich. The very idea seemed preposterous at the time. Fischer contended that a metal atom was surrounded and “held” by two organic groups. The scientific community had never before come across a molecule with that type of structure and had never even considered it possible. Yet this had to be the reason for the incredible stability of these orange-colored crystals, which only start to break down at a temperature of around 470°C.
The molecule soon came to be known as “ferrocene”. Fischer was awarded the Nobel Prize in 1973 for proving its sandwich structure – and for producing and investigating countless similar molecules with his laboratory team. The discovery of the sandwich compound fundamentally changed our understanding of how atoms can bond to each other. Fischer shared the Nobel Prize with Geoffrey Wilkinson. The English scientist had uncovered the structure of ferrocene in Harvard around the same time and, along with Professor Fischer, is regarded as a pioneer of organometallic chemistry.
It was Wilkinson who nicknamed this extraordinary compound the “molecular sandwich” – a fitting description by all accounts. The Nobel Committee actually presented ferrocene as an iron sphere between two slices of bread.
It is easy enough to create ferrocene. Heat pure iron with the hydrocarbon compound cyclopentadiene to approximately 300°C and you get ferrocene – or to give it its proper chemical name: dicyclopentadienyl iron (C10H10Fe). But what does actually happen during the reaction? Within the ring-shaped cyclopentadiene molecule, four of the five carbon atoms with double bonds are always tightly bound. Each one also has a hydrogen atom, but the fifth has two hydrogen atoms. When heat is applied, one of these two hydrogen atoms is released, and both rings draw the iron atom to the space between them.
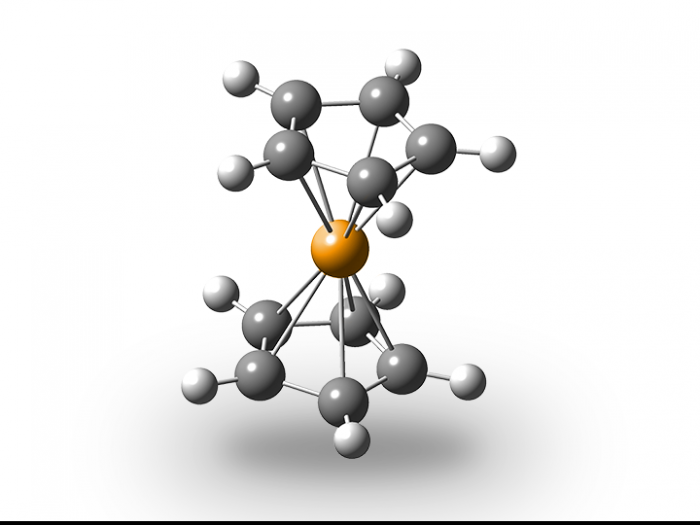
According to the theory at the time, each of the two cyclopentadienyl rings of ferrocene should have a negative charge – an electron which theoretically the ring must have snatched from the iron. But contrary to expectations, the iron and the two rings share the electrons so harmoniously that they outwardly present a completely uncharged, highly stable molecule. Fischer uncovered the unusual structure of the iron compound after conducting a variety of experiments, including a study of its magnetic behavior. But it was only after X-ray analysis conducted with his colleague Wolfgang Pfab that Fischer was able to confirm his discovery. The pair jointly published their findings – to the astonishment of the scientific community.

The “joy of experimenting in the pursuit of nothing other than knowledge” spurred Fischer on, and from 1964 to 1984, he held the professorship at TUM once held by Emil Erlenmeyer. Despite his primary commitment to basic research, Fischer’s work also has far-reaching impact in industry. Using ferrocene as a model, his research group and Wilkinson’s laboratory have produced hundreds of similar sandwich molecules, known as “metallocenes”. Some of these are proving to be effective catalysts, especially in the manufacture of plastics, where they speed up chemical reactions or even enable them in the first place. Fischer’s research thus laid the groundwork for TUM’s outstanding reputation in catalysis research.
„They did not prepare the first sandwich but they were the first to grasp the odd nature of the compound and its conceptual importance.“

Nobel committee,1973, at the prize ceremony for Ernst Otto Fischer und Geoffrey Wilkinson
Bild: © ®The Nobel Foundation: Photo: Lovis Engblom